Fill in the blanks! The first in China! Warm congratulations to Hunan Warner Pharmaceutical Co., LTD. Entecavir granules approved!
Release time:
2021-09-15
Source:
September 13, the State Food and Drug Administration announced that Hunan Warner Pharmaceutical Co., LTD. (stock code: 688799) entecavir particles (0.5mg) the first approved in the country! Entecavir granule is an improved dosage form suitable for children, which is developed according to the characteristics of children's medication and fills the gap in the market of children's dosage form for chronic hepatitis B in China.
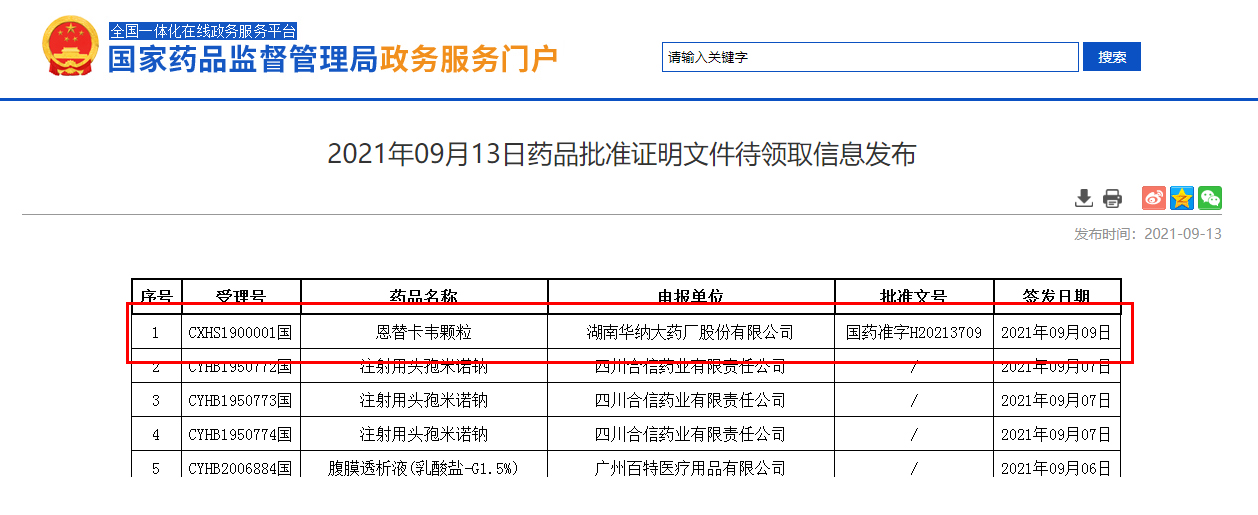
The variety completed by are biological "one-stop" clinical research: subsidiary shed with intelligent responsible for recruiting, first brought medicine SMO service, Jordan farmar ke data to perform data management and statistical analysis, nanning second people's hospital for clinical cooperation unit, the company the first project to work with the second people's hospital of nanning. This product analysis is exempt from on-site inspection.

In the post-4 +7 era, the country issued relevant guidelines for improved new drugs, which put forward directional suggestions for the research and development of improved new drugs. However, how to correctly understand the guidelines for improved new drugs and locate clinical development strategies has been perplexing enterprises.
Duzheng Bio focuses on providing one-stop service for clinical research of innovative drugs and generic drugs, and has significant advantages in early clinical research of innovative drugs, clinical research of improved new drugs, PPI drugs and other difficult preparation BE research fields. Through scientific and reliable scheme design, efficient recruitment and management of subjects, rich clinical resources, strict quality management and risk control system, and perfect information system guarantee, we help pharmaceutical enterprises solve clinical pain points, highlight clinical value, and help pharmaceutical industry innovation and development.



