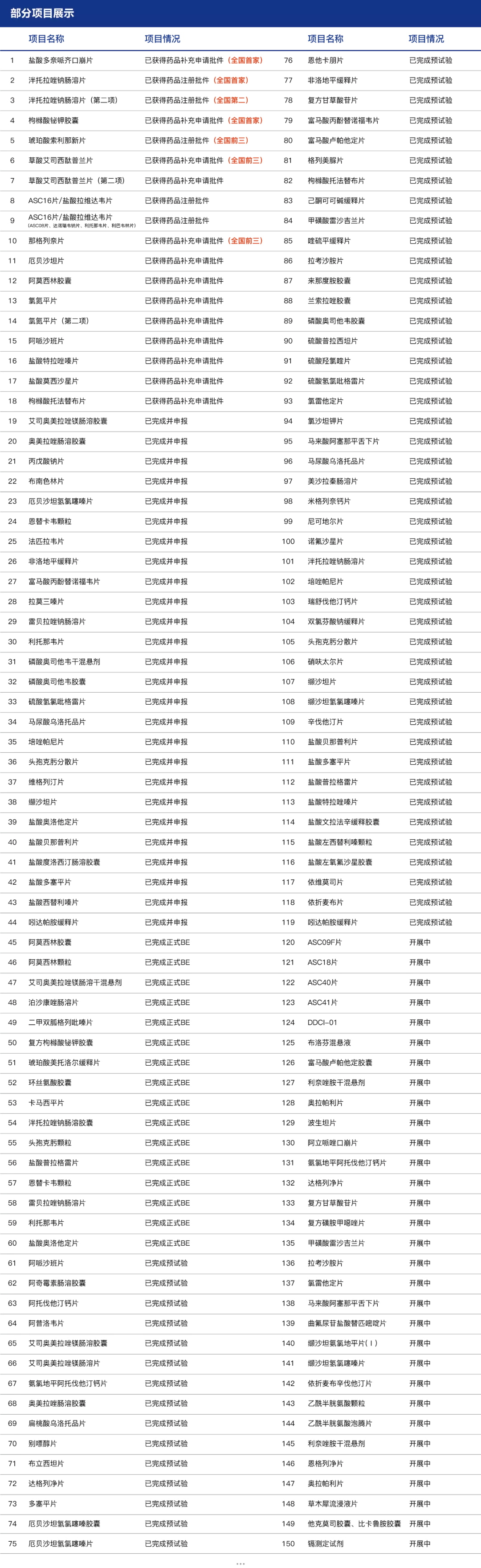
Congratulations to Hainan General Sanyo Pharmaceutical Co., LTD for the successful approval of vigliptin tablets
Release time:
2021-09-03
Source:
On September 3, sFDA announced that Hainan General Sanyo Pharmaceutical Co., LTD. Veogliptin tablets (50mg) successfully approved!
The variety completed by are biological "one-stop" clinical research: subsidiary shed with intelligent responsible for recruiting, first brought medicine SMO service, Jordan farmar ke data to perform data management and statistical analysis, mental health center in wuhan city to undertake clinical cooperation unit, the company with the first project of wuhan jingwei cooperation, has come to a successful conclusion.
Clinical and analysis of this product are exempt from on-site verification.
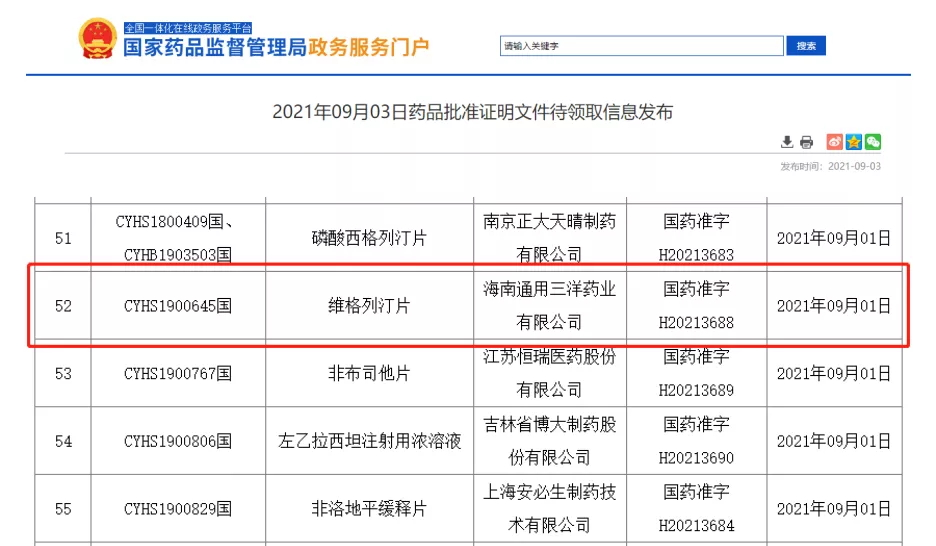
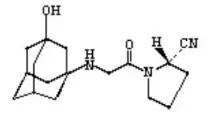
Vigagliptin is a novel oral hypoglycemic agent that inhibits dipeptidyl peptidase 4(DPP-4) and blocks the degradation of DPP-4 on glucagon-like peptide-1 (GLP-1) in vivo, thereby promoting insulin secretion, inhibiting glucagon secretion, and reducing fasting and postprandial blood glucose. Control blood glucose and reverse the deterioration of islet function in diabetic patients. At present, the oral often-released form of vigliptin has been included in the third batch of national collective procurement, making it the first dPP-4 inhibitor hypoglycemic drug to be adopted collectively.
As a "one-stop" service platform for clinical research, Duzheng Biological has helped many pharmaceutical companies successfully obtain approval. After "July 22", the company has carried out more than 300 clinical studies, more than 200 have been completed, more than 20 approved production, more than 70 times by the national and provincial bureau of inspection, the pass rate of 100%.