Duxact news warmly congratulates Zhejiang Haizheng Pharmaceutical Co., Ltd. on the first approval of faveravir tablets in China!
Release time:
2021-11-22
Source:
On November 19, the State Food and Drug Administration announced that "faviravir" (formerly known as "fapiravir") developed by Zhejiang Haizheng Pharmaceutical Co., Ltd. was officially approved by the State Food and Drug Administration for listing.
The "one-stop" clinical research on the bioequivalence of this variety of human body (postprandial test) is completed by Duzheng biology: the strategic cooperation unit Hunan maternal and child health hospital is responsible for clinical, Hutong intelligence is responsible for recruitment, first medicine is responsible for SMO services, and weight Ke data is responsible for data management and statistical analysis.
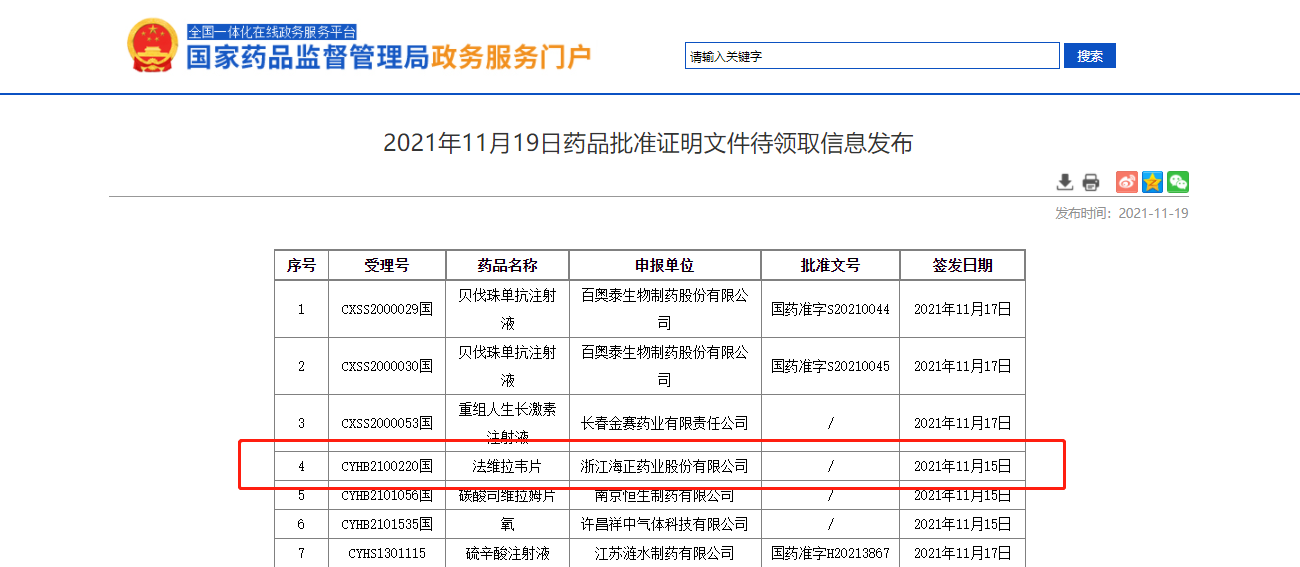
Faveravir tablets are used in the treatment of new and recurrent influenza. After continuous test and exploration in the early stage of this variety, it was entrusted to Duzheng biology. Successful cases make the market recognize Duzheng's strength. We will continue to adhere to the principle of "innovation and truth-seeking". Through scientific and reliable scheme design, efficient subject recruitment and management, rich clinical resources, strict quality management and risk control system, and perfect information system guarantee, we will help pharmaceutical enterprises solve clinical pain points and help the innovative development of pharmaceutical industry.


